当前位置:首页 / News / Company News
Suzhou Huiliao Bio Announces Another Good News in the Field of Veterinary mRNA Vaccines! Important progress has been made in the research and development of porcine deltacoronavirus mRNA vaccine.
时间:2024-08-22 14:06:58 文章来源:苏州慧疗生物
Suzhou Huiliao Biotechnology Co., Ltd.,简称“苏慧疗” (Suhuiliao), was established in May 2021 at the Suzhou Industrial Park 2.5 Industrial Park. It is a high-tech enterprise dedicated to independent innovation and the industrialization of nucleic acid drugs. Since its inception, Suhuiliao has been a pioneer in the field of international animal mRNA vaccine development, driven by its forward-looking vision. The company not only excels in mRNA technology but also holds over 40 foundational technology patents. It has built an innovative "trinity" technology platform that integrates mRNA design and synthesis, multi-technology delivery systems, and high-throughput microfluidic encapsulation. This platform lays a solid foundation for the research, development, and innovation of veterinary mRNA vaccines.
Porcine deltacoronavirus (PDCoV) is an emerging swine enteric coronavirus that poses a significant threat to newborn piglets, causing diarrhea, vomiting, dehydration, and even death. Since its first outbreak on U.S. pig farms in 2014, PDCoV has rapidly spread globally. Its destructive impact extends beyond animal health, profoundly affecting the swine industry economically. The high transmissibility and mortality rate of PDCoV not only disrupt the stability of pork supply but also jeopardize the livelihoods of farmers and the sustainable development of the entire industry chain.
In the face of this challenge, vaccination has become critically important. Traditional vaccine development is time-consuming and struggles to keep pace with viral mutations and epidemics. However, breakthroughs in mRNA vaccine technology offer a promising solution for PDCoV prevention and control. mRNA vaccines, known for their rapid response, high efficacy, and ease of production, provide the potential for swift development of vaccines targeting currently circulating strains. Moreover, they have been proven to offer strong protective efficacy.
----------------------------------------
Recently, Suzhou Huiliao Biotechnology Co., Ltd., in collaboration with the research team led by Dr. Li Bin from the Institute of Veterinary Medicine at the Jiangsu Academy of Agricultural Sciences, has made significant progress in the development of a PDCoV mRNA vaccine. The research findings, titled "A spike-based mRNA vaccine that induces durable and broad protection against porcine delta coronavirus in piglets," were published online in the internationally renowned virology journal Journal of Virology. This achievement marks an important milestone in the fight against PDCoV and highlights the potential of mRNA vaccine technology in addressing emerging animal diseases. This study is the first to report that the PDCoV mRNA vaccine has good immune protection effects and has commercial prospects. The research was supported by projects such as the "14th Five-Year Plan" key R&D program and the Jiangsu Province Agricultural Independent Innovation Fund.

The PDCoV S protein contains multiple neutralizing domains capable of inducing the production of neutralizing antibodies in the host. In this study, two mRNA vaccines based on the PDCoV S protein were designed: one encoding the full-length S protein and the other encoding a modified S protein (Se) with the transmembrane and hydrophobic regions removed. The optimized antigen-coding sequences were synthesized into mRNA through in vitro transcription and then encapsulated in lipid nanoparticles (LNP) to prepare the vaccines. Both mRNA vaccines exhibited excellent physical characteristics and high levels of antigen expression.
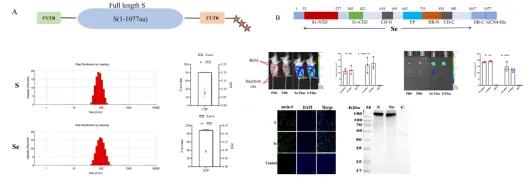
Figure 1. mRNA sequence design and LNP characterization of PDCoV S protein.
Our murine immunization trials revealed that mRNA vaccines encoding the full-length S protein elicited elevated levels of neutralizing antibodies and cellular immune responses, thereby demonstrating superior immunogenicity. These findings suggest that mRNA vaccines based on the PDCoV S protein can provide more effective immune potency.
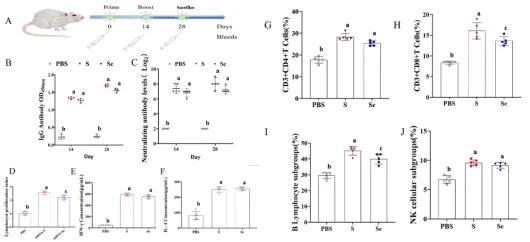
Figure 2. PDCoV S mRNA vaccine effectively activates the immune response in mice.
Subsequently, researchers immunized pregnant sows with the PDCoV S mRNA vaccine, with a PDCoV inactivated vaccine serving as a control. Antibody levels in both the sows and their piglets were assessed, and the piglets underwent a challenge protection test. The results demonstrated that the sows' serum contained high levels of neutralizing antibodies after the second immunization, while their colostrum contained substantial amounts of neutralizing antibodies and IgA mucosal antibodies. Following colostrum intake, the piglets' serum exhibited high levels of neutralizing antibodies and IgA antibodies, which persisted for up to three months. Upon challenge, none of the piglets in the mRNA vaccine group developed diarrhea, whereas one piglet in the PDCoV inactivated vaccine group experienced mild diarrhea. In contrast, all five piglets in the challenge control group developed persistent and severe diarrhea. Furthermore, the mRNA vaccine group showed significantly lower levels of viral shedding in rectal swabs, viral load in intestinal tissues, gross lesions, and microscopic lesions compared to the challenge control group (P < 0.05). These findings indicate that PDCoV S mRNA vaccine immunization of sows provides effective passive immune protection for piglets.
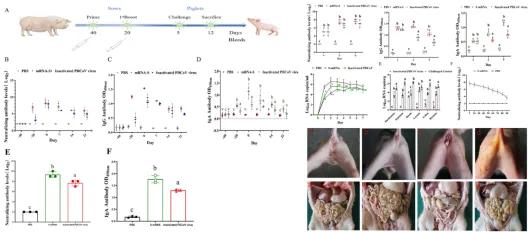
Figure 3. PDCoV S mRNA vaccine immunization of sows provides passive protection to piglets.
In summary, the PDCoV S mRNA vaccine developed by this team can induce high levels of neutralizing antibodies and activate cellular and mucosal immunity. The neutralizing antibodies induced by the vaccine can last up to 3 months. Immunizing pregnant sows provides newborn piglets with good passive immune protection, which can be used as a candidate vaccine for the prevention and control of PDCoV and has broad application prospects.
This significant progress in the development of the porcine deltacoronavirus mRNA vaccine marks a solid step forward in this cutting-edge field of technology. This achievement not only demonstrates the outstanding strength of Suhui Therapeutics in biomedical innovation but also brings new hope for the prevention and control of the global pig industry, indicating that we are accelerating towards a future of more efficient and safer animal health management.
#About Suzhou Huiliao #
Suzhou Huiliao Biotechnology is a high-tech enterprise engaged in the comprehensive provision of nucleic acid drug industrialization. The company's business spans the research and production of mRNA drugs, clinical trial design, data analysis, and other areas, with a focus on the development of mRNA technology-based infectious disease vaccines, cancer vaccines, cell therapies, protein replacements, and more.

-
慧疗生物荣登“2025中国新晋···
2025/03/14
-
慧疗生物完成近亿元A轮首关融资···
2025/01/22





